Introduction
The E2 reaction, denoting bimolecular elimination, is a singular step process where the breaking of carbon-hydrogen and carbon-halogen bonds concurrently results in the formation of a pi bond. This reaction is not characterized by the number of steps involved, but by its kinetics, being bimolecular, it is influenced by both the alkyl halide and the base. The formation of a pi bond necessitates the antiperiplanar arrangement of the leaving groups. This antiperiplanar transition state has a staggered conformation, which is lower in energy compared to a synperiplanar transition state that has an eclipsed conformation. A strong base is typically used in E2 reactions, which must be potent enough to remove a weakly acidic hydrogen. The creation of the pi bond requires the carbons to change their hybridization from sp3 to sp2. The rate-determining step involves the weakening of the C-H bond. Lastly, E2 reactions compete with SN2 reactions if the base can also function as a nucleophile.
Reaction

-
The E2 elimination is a 1-step reaction
-
The hydrogen being removed by a base and the leaving group must be anti-periplanar (on the same plane, but on opposite sides)
Mechanism

-
A base removes a proton that’s anti-periplanar to the leaving group in one step, with no intermediates.

-
Large, sterically-hindered bases, such as tert-butoxide, will abstract the more easily-accessible proton to produce the less substituted alkene (Hofmann product)
Bases

-
Non-bulky, charged anions, such as those shown above, can carry out both SN2 and E2, so mixtures of products typically occur as there isn’t complete selectivity for one over the other.
-
Sterically-hindered bases, such as those shown above on the right side, are too hindered to act as nucleophiles and instead act solely as bases.
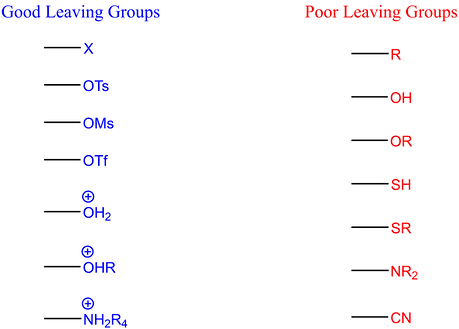
Leaving Groups
The leaving group pattern is the same as for SN2. Whatever is a good leaving group for SN2 is also a good leaving group for E2.
Zaitsev vs Hofmann Eliminations

-
Small, non-sterically-hindered bases, such as hydroxides or small alkoxides, will produce the more highly-substituted alkenes (Zaitsev product).

-
Large, sterically-hindered bases, such as tert-butoxide, will abstract the more easily-accessible proton to produce the less substituted alkene (Hofmann product)
Keywords
bimolecular elimination | e2 | nucleophile | leaving group | electrophile | base